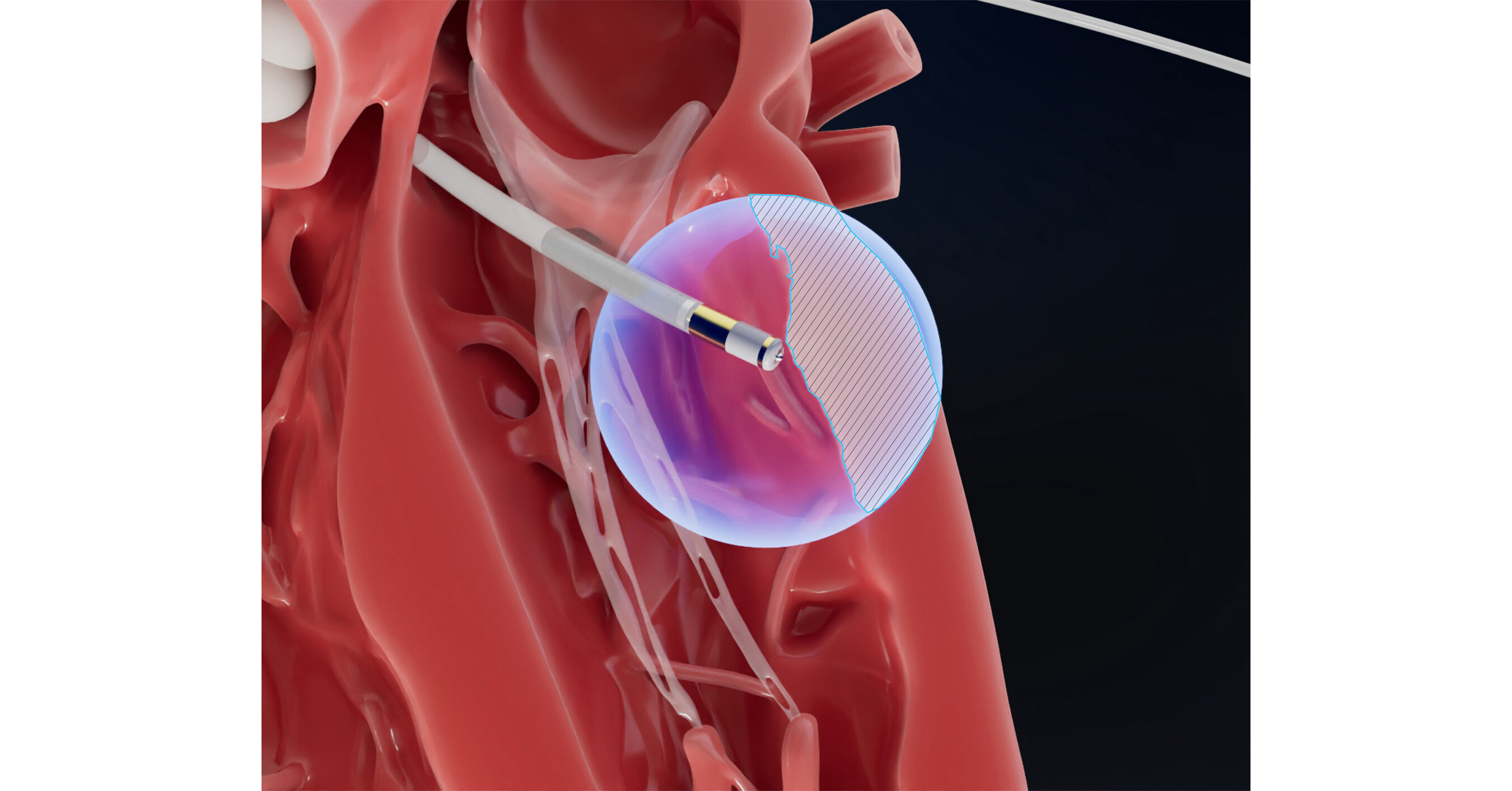
Field Medical Presents Positive 6-Month First-in-Human Data for PFA System in Ventricular Tachycardia
AI-Generated Summary
Field Medical announced the presentation of positive 6-month first-in-human data for its FieldForce™ Pulsed Field Ablation (PFA) System in treating scar-related ventricular tachycardia at the VT Symposium. The company also highlighted its recent Breakthrough Device Designation and entry into the FDA TAP Pilot Program for its VT indication. Additionally, Field Medical announced strategic leadership changes to strengthen its focus on innovation and growth.
In a nutshell
This data provides crucial evidence for the potential of PFA technology in a challenging cardiac arrhythmia, offering a new therapeutic avenue for healthcare professionals and patients. The FDA's Breakthrough Device Designation further validates the innovative approach and accelerates its path to market.
Source: PR Newswire UK