FDA Fast-Tracks Controversial Autism-Related Drug Approval with GSK, Drawing Expert Scrutiny
AI-Generated Summary
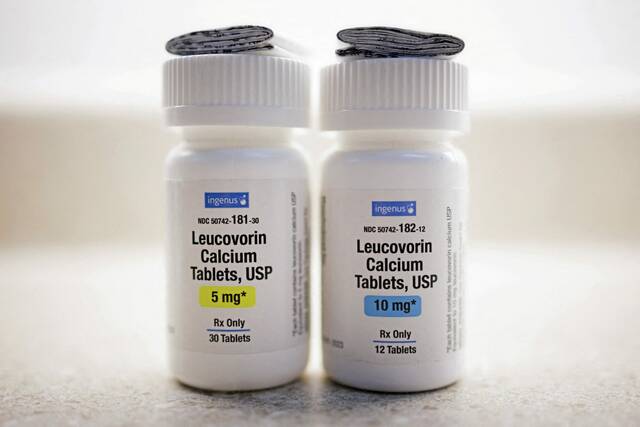
The FDA, with assistance from GSK, is fast-tracking the approval of leucovorin for an autism-related disorder, a move championed by Health Secretary Robert F Kennedy Jr. This unusual regulatory maneuver bypasses extensive clinical trials or label updates for generic versions, drawing significant skepticism from academics and medical experts regarding the robustness of supporting evidence. The decision is seen as a policy win for the Trump administration, despite concerns over scientific rigor.
In a nutshell
This piece highlights the increasing intersection of political influence and regulatory processes within the healthcare industry, raising critical questions for pharmaceutical professionals and regulatory affairs specialists about evidence standards and approval precedents. The unusual fast-track mechanism could set a precedent for future drug approvals, demanding close attention from our audience.
Source: Pittsburgh Tribune-Review



